You are here
X: One final study for persulfate decay amid a whirl of writing.
During my last week as a summer undergraduate intern at CMOP, I managed to squeak in one more persulfate decay study. All of my solutions thus far have been fairly pure, filtered samples. For this study, I wanted to simulate (to an extreme) the effect of metal ballast tanks. I gathered three kinds of solid iron samples: Fluka filings, cast iron powder, and electrolytic grade iron powder. With 5 g iron in glass VOA vials with 40 mL of DI or Instant Ocean water in each, we aggitated them all weekend to simulate natural oxidation.
Monday morning I arrived to discover a variety of rusty solutions, which we used Tuesday in persulfate decay studies. With adequate blanks and some replicates, this study was completely a shot in the dark. We designed the experiement to have some solutions with iron solid and some with out. Due to the large dilution factor when analyzing samples for persulfate concentration, the rusty background did not interfere much with our method. We observed iron quickly removes active persulfate from solution at high temperatures, the excess persulfate decays with usual kinetics in saline and DI waters, respectively. Samples with solid iron had almost no persulfate present, but samples of solutions exposed to iron had reduced absorbances, but the usual pattern of decay. Though the type of iron had some effects on decay, the salinity effect was the primary determinant of persulfate decay (shown below).
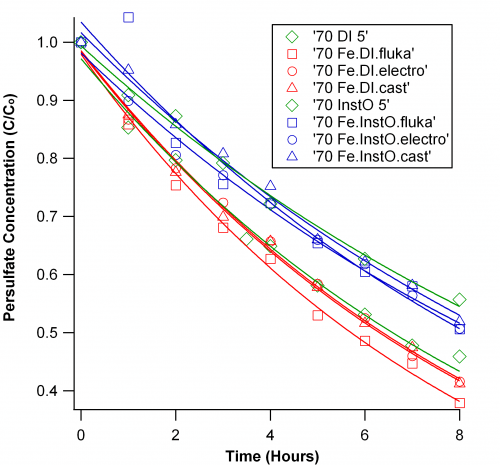
As a side note, I finally discovered how to put these studies in a positive light; talking only about decay can become rather pessimistic. That is, these studies have been to assess the stability of persulfate in aqueous solutions. The lifetime of the active oxidant has major bearing on its effectiveness as a disinfectant. Even with our accelerated decay studies at high temperatures, persulfate is realatively stable in aqueous solutions, and therefore remains a strong candidate for ballast water disinfection.
I completed a thorough SOP (Standard Operating Procedure) documenting my method for persulfate decay studies. I enjoyed making everything crystal clear and found great satisfaction reflecting on my minor innovations. I included a comprehensive discussion about using the Gilford Stasar III; apparently this summer I became the most experienced on the instrument in the entire Tratnyek lab.
My capstone assignments to the CMOP intership included a presentation and a final paper. I've attached the presentation. The paper is unusually comprehensive, as it discusses the development of the method and the conclusions of the stability studies. Both were excellent exercises in developing the results from my project this summer.
This has been a special opportunity that I value for the following reasons:
- Experience with rather autonomous scientific research (I even had my own office connected to the lab!)
- Gaining a better understanding for the caliber of graduate school
- Developing marketable skills and professionalism
- Fostering personal growth.
On top of that, it has been in a friendly, welcoming atmosphere with call to excellence. Thank you to all that have been a part of it!
| Attachment | Size |
|---|---|
| 1.09 MB |



