You are here
IX: The Final Countdown: conclusions about rate constants and the future of persulfate chemistry
Here are some thoughts from/for my final paper. It gives some thorough discussion of my results. The data for additional sulfate/salinity/natural water studies are not included, but feel free to contact me if you have questions:
Temperature is the overlying variable affecting persulfate decay; our data is consistent with the fundamentals of kinetics by showing faster decay at higher temperatures. The rate constants from our systematic observations are shown (below) in a modified Arrhenius plot that summarizes the data for all temperature, water type and initial persulfate concentration variables.
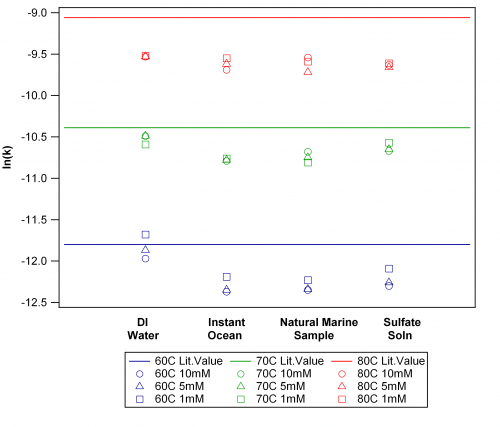
Natural logarithm of rate constants from first order decay of persulfate in un-buffered aqueous solutions. Temperature is indicated by color, solvent is shown on the bottom axis, and initial concentrations are given in the legend.
Key Observations:
- Our data reports slower decay than the predicted literature values for each temperature (Johnson et al. 2008). Since the literature data was based on persulfate decay in DI water, the deviation in our DI samples, which increased with temperature, suggests that the extrapolation of the published equation from the Arrhenius plot may not accurate. Alternatively, our un-buffered solution may be subject to pH variability that would slow decay; our studies measured the change in pH but did not attempt to control it, thereby simulating oceanic conditions. Regardless, the unresolved discrepancy does not detract from the other observed trends.
- Salinity has another clear effect on persulfate decay. The saline and sulfate solutions are unanimously slower decay than predicted values, but additionally they are slower than DI water.
- Instant Ocean and natural ocean samples show equivalent rate constants regardless of temperature.This will simplify our future studies by assuming Instant Ocean solutions effectively simulate natural ocean water, allowing more control of percent composition, natural organic matter, and salinity.
The more recent water-variable investigations reiterated this salinity trend; the relationship may even extend to ionic strength. There is undoubtedly slower decay with increasing salinity, but it is not a linear relationship. The mere presence of ions in solution causes a dramatic effect in slowing decay. Though it is not a saline solution, the sulfate study gave a similar conclusion: the presence of sulfate slows decay, but in this case there is little differentiation between concentrations. The comparison between river water with low conductivity and natural seawater is analogous to the respective kinetics of DI and saline waters. The evidence of this salinity effect amid other variables reasserts its prominence, and the sulfate studies indicate that the effect may be more widely applied as ionic strength.
Inconclusive observations:
- Varying the initial persulfate concentration did not have a notable effect on persulfate decay, but there is a slight differentiation among the 1 mM persulfate samples at 60˚C. If persulfate is used as ballast water treatment in more dilute doses, those doses would require further stability studies.
- Our natural water samples exhibited faster decay in filtered samples over unfiltered samples. This occurrence may be an exception, but a rigorous testing of persulfate decay in relation to natural oxidant demand will have a strong effect on the effectiveness of persulfate as a ballast water treatment.
FINALLY...
- Temperature is the primary variable governing persulfate decay, but there is strong evidence that increased salinity and ionic strength correspond to slower decay.
- It may be necessary to revisit stability studies as we learn the adequate dosage of persulfate for disinfecting ballast water.
- Persulfate remains a strong candidate for chemical ballast water treatment amid anticipation of studying its effectiveness as a disinfectant in seawater.
*Rewritten 8/13/2010 after the original posting was lost in cyberspace.



